
Ivermectin May Defeat Cancer and Other Common Chronic Diseases of Aging
If you think Big Pharma had good reasons to censor ivermectin during COVID-19 how about now when we know it is likely effective against all chronic diseases associated with aging?
This Substack recently wrote about the powerful anticancer properties of Fenbendazole:
PetDazole: Pharmaceutical Grade Pure Fenbendazole
Just like they went after one of the very best cures for PSYOP-19 in Ivermectin… …the Medical Industrial Complex does not want the truth to come out about a powerful cancer cure in Fenbendazole. Thanks to the deployment of the DEATHVAX™, we are now seeing parabolic increases in “turbo cancers.” Those responsible for these slow kill bioweapon injections…
I also mentioned in passing that one of reasons Ivermectin was so viciously maligned and suppressed was that if society were taking it to cure PSYOP-19 one of the side effects would be “sudden” plummeting cancer rates, and thus BigPharma et al. went all out to destroy this Nobel prize miracle drug.
What would happen if one did a combination therapy for both the prevention and treatment of cancer using BOTH Ivermectin and Fenbendazole? The synergistic pairing would be far more effective than just using one of these miraculous drugs.
For many years now the cure for cancer was in plain sight right in front of everyone all this while, yet society was socially engineered to mindlessly “Trust the Science” such that they would undergo incredibly expensive (read: exorbitant profit margins) medical treatments that are toxic and deadly, instead of taking two inexpensive (read: minuscule profit margins) drugs that have zero adverse side effects.
It also turns out that Ivermectin is a powerful anti-aging drug!
This is precisely why the war on Ivermectin (and soon Fenbendazole) has only just begun…
Dr. Paul Marik recently quoted a prospective clinical trial where the participants were given 4000 international units of Vitamin D, omega 3’s and told to exercise and the risk of cancer dropped 50%.
In another post, Dr. Marik says it is highly unlikely cancer is genetically determined.
I wanted to talk about both in this post.
In July 1994, I published a new theory of cancer in Molecular Carcinogenesis which implied while tumors were genetically determined, the malignant nature of tumors (ie., the thing we call cancer) was not [1].
This notion that malignancy was a phenotype and not a genotype was heretical at the time and so the paper was ignored (only the editors of Molecular Carcinogenesis and myself were excited). It was an exciting idea because it meant one can control the malignant potential of tumors pharmacologically. No more need for slash and burn, which I have always regarded as barbaric.
However, subsequently it became widely accepted that the malignant phenotype of cancers called ‘epithelial mesenchymal transition’ (EMT) was real [2]. So I was vindicated, although it seems no one noticed except for myself.
The reason I had proposed cancer as a phenotype was that I had just finished the characterization of the 67 kD alpha-fetoprotein (AFP) receptor for my Ph.D. thesis [3]. The AFP receptor (AFPr) was expressed on macrophages and highly overexpressed on the common cancers the adenocarcinomas (breast, prostate, lung, colon, etc) implying a dual role in immunosuppression of the host and in tumor malignant potential. In fact I wrote the theory to explain how immunosuppression of the host relates to tumor malignant potential.
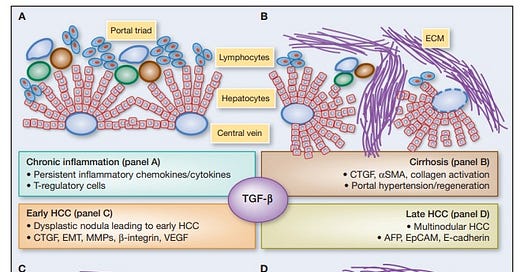
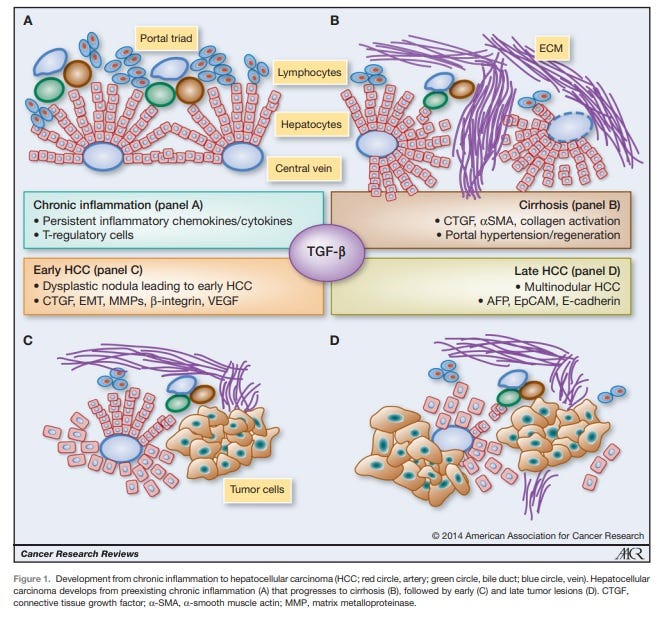
Figure 1. From Giannelli G et al, Cancer Research 2014 [4]. AFP is recognized as a malignancy progression factor in a very common cancer in the world, hepatocellular carcinoma (HCC) often associated with a viral origin.
Thus, the malignant potential of tumors known as cancer was amenable to pharmacological intervention.
AFP exists in active and inactive states. Things that bind to and inactivate AFP (zinc, DHEA, flavonoids etc) are entities that may help promote innate immunity (of macrophages) and which also diminish the malignant potential of tumors.
At the time I called this malignant phenotype of cancers “anti-cellular senescence” [1]. This was because the tumor was refractory to new signaling and thus changes were not visible, because AFP binding to the AFPr on tumors blocked incoming signals. So it seemed the tumor did not age.
While the malignant phenotype is now recognized as ‘epithelial-mesenchymal transition’ (EMT), fortunately Dr. Robert Weinberg has also defined it to block senescence [5] or is also a phenotype involving anti-cellular senescence (whew!).
Then in 2015, I wrote the new immunosenescence paradigm of macrophages published in Discovery Medicine which attempts to explain the cause of chronic illness associated with aging including diseases such as cancer and cardiovascular diseases [6]. Subsequently I validated this paradigm specifically for explaining the initiation and progression of cardiovascular diseases [7] (it is not cholesterol but elevated stress does increase cholesterol).
Figure 2. The New Immunosenescence Paradigm of Macrophages
defined as the failed (lytic) release of protector HERV-K102 from foamy macrophages. When the DHEA/cortisol ratio is low, there is a higher risk of immunosenescence when the host encounters a virus due to inadequate levels of DHEA to bind and inactivate AFP. We reported in 1994 that AFP blocks apoptosis of macrophages [8].
P53 along with TGF-beta, represses AFP expression, and p53 is a tumor suppressor commonly deleted or at least dysfunctional in tumors/cancers.
ZBTB20 is a zinc finger credited with post-natal down-regulation of AFP. However, when functional p53 is absent, it upregulates AFP [9].
Did you know that ZBTB20 is required for the induction of NFKB1 [10] such as when SARS-CoV-2 infects foamy macrophages in vivo by ADE [11]which contributes to cytokine storm? AFP is significantly upregulated by SARS-CoV-2 infection at the protein and mRNA level in cell lines [12].
Referring back to Figure 2, AFP antagonists reverse and prevent immunosenescence. Immunosenescence causes age associated chronic diseases [6,7]. This means AFP antagonists like zinc, flavonoids, DHEA/7ketoDHEA, Vitamin D over 60 ng/ml, and now also ivermectin [13] are likely AFP antagonists which are predicted to reduce the risks of age-associated chronic illness including cancer and cardiovascular disease, etc..
There are now in addition to many reports on how ivermectin behaves as a potent antiviral, emerging evidence for its reversion of the malignant phenotype particularly by inducing apoptosis [14] and blocking metastasis [15].
So if you think the war on ivermectin is over, in fact, it is just getting STARTED.
REFERENCES
Laderoute MP. A new perspective on the nature of the cancer problem: anti-cellular senescence. Mol Carcinog. 1994 Jul;10(3):125-33. doi: 10.1002/mc.2940100303.
Zhang Y, Weinberg RA. Epithelial-to-mesenchymal transition in cancer: complexity and opportunities. Front Med. 2018 Aug;12(4):361-373. doi: 10.1007/s11684-018-0656-6.
Laderoute MP. The Characterization of a Novel, Widespread, PNA-Reactive Tumor Associated Antigen: the Alpha-fetoprotein Receptor/Binding Protein. Ph.D. Thesis. The University of Alberta. Canada 1991, pp 256. https://era.library.ualberta.ca/items/6f548eb6-49a2-456c-b472-41f68976077f.
Giannelli G, Villa E, Lahn M. Transforming growth factor-β as a therapeutic target in hepatocellular carcinoma. Cancer Res. 2014 Apr 1;74(7):1890-4. doi: 10.1158/0008-5472.CAN-14-0243.
Weinberg RA. Twisted epithelial-mesenchymal transition blocks senescence. Nat Cell Biol. 2008 Sep;10(9):1021-3. doi: 10.1038/ncb0908-1021.
Laderoute MP. A new paradigm about HERV-K102 particle production and blocked release to explain cortisol mediated immunosenescence and age-associated risk of chronic disease. Discov Med. 2015 Dec;20(112):379-91.
Laderoute M. The paradigm of immunosenescence in atherosclerosis-cardiovascular disease (ASCVD). Discov Med. 2020 Jan-Feb;29(156):41-51.
Laderoute MP, Pilarski LM. The inhibition of apoptosis by alpha-fetoprotein (AFP) and the role of AFP receptors in anti-cellular senescence. Anticancer Res. 1994 Nov-Dec;14(6B):2429-38.
To JC, Chiu AP, Tschida BR, Lo LH, Chiu CH, Li XX, Kuka TP, Linden MA, Amin K, Chan WC, Bell JB, Moriarity BS, Largaespada DA, Keng VW. ZBTB20 regulates WNT/CTNNB1 signalling pathway by suppressing PPARG during hepatocellular carcinoma tumourigenesis. JHEP Rep. 2020 Dec 19;3(2):100223. doi: 10.1016/j.jhepr.2020.100223.
Liu X, Zhang P, Bao Y, Han Y, Wang Y, Zhang Q, Zhan Z, Meng J, Li Y, Li N, Zhang WJ, Cao X. Zinc finger protein ZBTB20 promotes Toll-like receptor-triggered innate immune responses by repressing IκBα gene transcription. Proc Natl Acad Sci U S A. 2013 Jul 2;110(27):11097-102. doi: 10.1073/pnas.1301257110.
Ren X, Wen W, Fan X, et al. COVID-19 immune features revealed by a large-scale single-cell transcriptome atlas. Cell. 2021 Apr 1;184(7):1895-1913.e19. doi: 10.1016/j.cell.2021.01.053.
Appelberg S, Gupta S, Svensson Akusjärvi S, et al. Dysregulation in Akt/mTOR/HIF-1 signaling identified by proteo-transcriptomics of SARS-CoV-2 infected cells. Emerg Microbes Infect. 2020 Dec;9(1):1748-1760. doi: 10.1080/22221751.2020.1799723.
Laderoute M. Ivermectin may prevent and reverse immunosenescence by antagonizing alpha-fetoprotein and downmodulating PI3K/Akt/mTOR hyperactivity. Open Heart. April 29, 2021. https://openheart.bmj.com/content/8/1/e001655.responses#ivermectin-may-prevent-and-reverse-immunosenescence-by-antagonizing-alpha-fetoprotein-and-downmodulating-pi3k-akt-mtor-hyperactivity.
Tang M, Hu X, Wang Y, Yao X, Zhang W, Yu C, Cheng F, Li J, Fang Q. Ivermectin, a potential anticancer drug derived from an antiparasitic drug. Pharmacol Res. 2021 Jan;163:105207. doi: 10.1016/j.phrs.2020.105207.
Jiang L, Sun YJ, Song XH, Sun YY, Yang WY, Li J, Wu YJ. Ivermectin inhibits tumor metastasis by regulating the Wnt/β-catenin/integrin β1/FAK signaling pathway. Am J Cancer Res. 2022 Oct 15;12(10):4502-4519.
They want you dead.
Do NOT comply.

















I have put in an order for Petmectin for my large dog that weighs the exact same as me. Let's see if it is delivered from the US.
Anticancer Potential of Repurposed Drugs and Natural Compounds: A Focus on Ivermectin, Fenbendazole, Quercetin, Vitamins C and D3, and Curcumin
By Sid Belzberg
Introduction
In the evolving landscape of anticancer therapies, the search for novel agents often uncovers unexpected candidates. Among these are drugs like Ivermectin and Fenbendazole, primarily used as antiparasitics, along with naturally occurring substances such as Quercetin, Vitamins C and D3, and Curcumin. Intriguingly, these compounds have demonstrated anticancer properties in vitro and in vivo, but the path to human trials has been slow and challenging, largely due to economic considerations in the repurposing of patent-expired drugs and natural compounds.
Repurposed Drugs and Natural Compounds as Anticancer Agents
Both Ivermectin and Fenbendazole have shown anticancer potential in preclinical studies, with reported effects including cytotoxicity to cancer cells and inhibition of tumor growth. The mechanisms underlying these effects appear to involve disruption of critical cellular processes, leading to cancer cell death.
Similarly, certain flavonoids and vitamins have shown promising anticancer properties. Quercetin, a flavonoid found in many fruits and vegetables, exhibits antioxidant, anti-inflammatory, and antiproliferative activities that can potentially inhibit cancer progression. Vitamins C and D3 have also shown anticancer potential, with Vitamin C inducing oxidative stress in cancer cells, and Vitamin D3 modulating cellular growth and differentiation. Curcumin, the active component of turmeric, has also demonstrated broad anticancer effects, potentially impacting multiple signaling pathways involved in cancer.
Economic Challenges in Drug and Natural Compound Repurposing
Despite these encouraging findings, the progression towards clinical trials for these compounds as anticancer treatments has been hindered. This slow pace can be attributed largely to economic constraints. Developing or repurposing a drug is a high-cost, time-intensive process, with clinical trials alone demanding significant financial and human resources.
For Ivermectin and Fenbendazole, their status as off-patent drugs allows production by multiple manufacturers, reducing the potential for return on investment for companies funding research into their anticancer uses. For natural compounds like Quercetin, Vitamins C and D3, and Curcumin, the inability to patent these substances similarly lowers the financial incentive for pharmaceutical companies to invest in extensive research and development.
The Case for Drug and Natural Compound Repurposing
Despite these challenges, the repurposing of these compounds carries potential advantages that justify further exploration. Since the safety and pharmacokinetic profiles of these substances are well-known, their development as anticancer agents could be faster and less expensive than for new drugs. Furthermore, the successful repurposing of these compounds could provide a cost-effective way to expand anticancer treatments, possibly improving patient outcomes while reducing healthcare costs.
Conclusion
While the economic aspects of drug development are a crucial consideration, they should not impede the pursuit of potential life-saving treatments. The cases of Ivermectin, Fenbendazole, Quercetin, Vitamins C and D3, and Curcumin highlight the need for alternative funding models and regulatory strategies to support the repurposing of off-patent drugs and natural compounds. Potential solutions could include public-private partnerships, non-profit or government funding, modifications to patent laws, or innovative models of drug development. By exploring such alternatives, we can ensure that the potential therapeutic benefits of these compounds are fully explored and capitalized on, irrespective of their economic attractiveness to pharmaceutical companies.
References
1 Caly, L., Druce, J.D., Catton, M.G., Jans, D.A., & Wagstaff, K.M. (2020). The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Research, 178, 104787.
2 Pourgholami, M.H., Khachigian, L.M., Fahmy, R.G., Badar, S., Wang, L., Chu, S.W., et al. (2010). Albendazole inhibits endothelial cell migration, tube formation, vasopermeability, VEGF receptor-2 expression and suppresses retinal neovascularization in ROP model of angiogenesis. Biochemical and Biophysical Research Communications, 397(4), 729-734.
3 Granja, A., Pinheiro, M., Reis, S. (2020). Epigallocatechin Gallate Nanodelivery Systems for Cancer Therapy. Nutrients, 8(5), 307.
4 Padayatty, S.J., Sun, A.Y., Chen, Q., Espey, M.G., Drisko, J., & Levine, M. (2010). Vitamin C: Intravenous Use by Complementary and Alternative Medicine Practitioners and Adverse Effects. PLoS ONE, 5(7), e11414.
5 Garland, C.F., & Garland, F.C. (1980). Do sunlight and vitamin D reduce the likelihood of colon cancer? International Journal of Epidemiology, 9(3), 227-231.
6 Hatcher, H., Planalp, R., Cho, J., Torti, F.M., & Torti, S.V. (2008). Curcumin: From ancient medicine to current clinical trials. Cellular and Molecular Life Sciences, 65(11), 1631-1652.
7. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5835698/. Mandy Juarez,1 Alejandro Schcolnik-Cabrera,1 and Alfonso Dueñas-Gonzalez2 The multitargeted drug ivermectin: from an antiparasitic agent to a repositioned cancer drug