
The journey of evidence-based medicine, particularly in the treatment of complex diseases such as cancer and Alzheimer’s, is marked by pivotal shifts in approach and philosophy, many of which can be traced back to the early 20th century.
The Rockefeller Foundation's involvement in the 1930s set the stage for profound changes in medical research and practice, emphasizing a model that would eventually favor pharmaceutical interventions over integrative and comprehensive approaches. This transformation has had lasting implications, often to the detriment of patients facing diseases, where non-pharmacological interventions could play a critical role.
In the realm of cancer treatment, this paradigm is exemplified by the predominant reliance on chemotherapy, a standard approach that overlooks the potential benefits of dietary interventions aimed at countering the Warburg effect—the altered metabolism of cancer cells favoring glycolysis even in the presence of ample oxygen.
Chemotherapy: Fraudulent and Deadly?
by Dr. Vernon Coleman Patients who are diagnosed with cancer find themselves in a state of shock. And yet, while in a state of shock, they find themselves needing to make a number of vital decisions very quickly. One of the big questions is often this one: “Should I have chemotherapy?”
This oversight underscores a broader issue in the medical community's approach to complex diseases: a tendency to focus on reductive, focused treatment rather than addressing all underlying systemic causes and promoting overall health through integrative strategies.
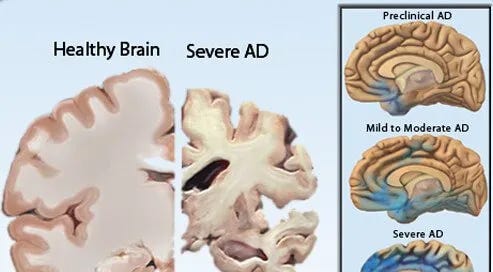
Alzheimer’s Disease presents a similar challenge where the prevailing focus has been on pharmaceutical interventions, often at the expense of exploring the full potential of dietary and lifestyle modifications.
The metabolic dysfunction hypothesis of Alzheimer’s, akin to the Warburg effect in cancer, suggests that addressing glucose metabolism and insulin resistance through diet could significantly impact disease progression. Yet, the integration of such approaches into standard treatment protocols remains limited.
The historical emphasis on a reductionist, organ-limited and medication-first model in evidence-based medicine has led to missed opportunities for more comprehensive, integrative care strategies.
When exploring the potential of compounds like allulose, fenbendazole, and ivermectin in treating Alzheimer’s, it is crucial to reflect on the broader context of the general approach to disease treatment. Revisiting the roots of evidence-based medicine and its evolution over the past century elucidates a more integrated approach that combines pharmaceutical interventions with dietary and lifestyle modifications to address the complex needs of patients with cancer, Alzheimer’s, and other chronic diseases.
A multifaceted treatment approach to AD
Allulose: Targeting Metabolic Dysfunction
Allulose's potential to enhance mitochondrial function and reduce insulin resistance positions it as a valuable component in treating the metabolic aspects of Alzheimer's Disease. By improving glucose metabolism and energy production within neurons, allulose could help mitigate the cognitive decline associated with metabolic dysfunction in AD.
Fenbendazole: Combating Infectious Components
The anti-Candida properties of fenbendazole introduce a novel angle for Alzheimer's treatment, targeting the infectious hypothesis of AD. By reducing fungal load and potentially lowering inflammation associated with infections, fenbendazole could contribute to preserving neuronal health and function.
DEMENTIA & ALZHEIMER'S CURE: Fungal Infection in the Brain Produces Effects Similar to Alzheimer's
A recent article by The Epoch Times posited that fungal infections may be the cause of neurodegenerative diseases. A team of researchers at Baylor College of Medicine has discovered that when the brain is infected with a common fungus, it changes in ways similar to those seen in Alzheimer’s disease. The
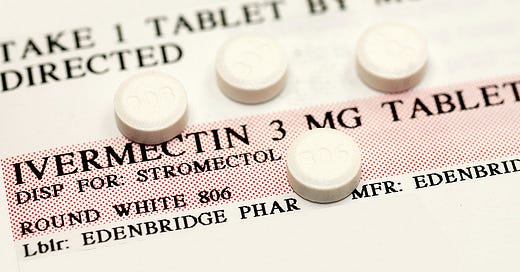
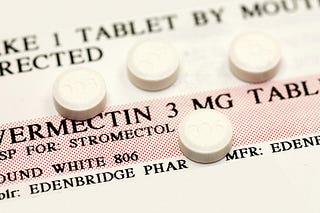
Ivermectin: Addressing Tauopathy and Hyperphosphorylation
Ivermectin's ability to prevent tau hyperphosphorylation and its potential neuroprotective effects offer a direct approach to addressing the core pathology of Alzheimer's Disease. By inhibiting the formation of neurofibrillary tangles (plaques), ivermectin could slow the progression of neurodegeneration in AD.
Alzheimer's as a Metabolic Disorder
The notion of Alzheimer's Disease functioning as a metabolic disorder, specifically termed "Type 3 diabetes," represents a paradigm shift in the understanding of this neurodegenerative condition.
This perspective underscores the critical role of metabolic dysfunction, particularly insulin resistance and impaired glucose metabolism, in the pathogenesis of AD. Here, the foundational elements of this hypothesis are explored along with its implications for neuronal health, and the potential avenues it opens for therapeutic intervention.
Insulin Resistance & Glucose Hypometabolism in the Brain
Central to the metabolic dysfunction hypothesis is the observation that brain cells in individuals with Alzheimer's show a marked decrease in their ability to utilize glucose efficiently.
Glucose is the primary energy source for the brain, fueling its complex activities under normal conditions. However, in AD, the brain's glucose metabolism is significantly impaired, leading to energy deficits that compromise neuronal function and contribute to cognitive decline. This impairment is akin to insulin resistance observed in diabetes, where cells fail to respond to insulin's signal to take up glucose from the bloodstream, despite its abundance.
Moreover, insulin not only regulates glucose metabolism but also plays a vital role in neurotransmitter signaling, learning, and memory. Insulin dysregulation in the brain can therefore disrupt these critical processes, further contributing to the symptoms of Alzheimer's.
The Brain's Metabolic Flexibility & Ketone Bodies
Another aspect of Alzheimer's as a metabolic disorder is the brain's reduced metabolic flexibility—the ability to switch between glucose and alternative fuel sources, such as ketone bodies, when glucose availability is low.
In the context of insulin resistance and glucose hypometabolism, the brain's ability to utilize ketone bodies derived from fatty acids becomes increasingly important. Ketone bodies can provide a more efficient energy source for neurons, potentially bypassing the metabolic deficiency seen in glucose utilization.
Therapeutic Implications
The metabolic hypothesis of Alzheimer's Disease opens new therapeutic avenues focused on restoring metabolic balance and enhancing energy metabolism in the brain.
Strategies such as dietary interventions (e.g., ketogenic diets), which increase the availability of ketone bodies, and the use of insulin sensitizers are being explored for their potential to improve cognitive function in AD patients. Additionally, compounds that can enhance mitochondrial function and energy metabolism directly within neurons could offer significant benefits.
The Role of Allulose
In this metabolic context, allulose - a rare sugar with a unique profile - emerges as a compound of interest. Allulose has been shown to exert several beneficial effects on metabolic health, such as reducing insulin resistance and enhancing fat oxidation, without contributing to the glucose load that could exacerbate insulin resistance in neurons. Its potential to enhance mitochondrial function could directly support the energy demands of brain cells, offering a novel approach to mitigating the metabolic dysfunction observed in Alzheimer's Disease.
Viewing Alzheimer's Disease through the lens of metabolic dysfunction not only enriches the understanding of its complex pathology but also broadens the horizon for developing more effective treatments. By targeting the metabolic disfunction at the heart of Alzheimer's, interventions such as allulose supplementation could pave the way for innovative strategies to combat this challenging condition, offering hope for improved outcomes in patients affected by this form of "brain diabetes."
Mitochondrial Function & Glucose Metabolism in Alzheimer's Disease
Allulose is a rare sugar, structurally similar to fructose yet functionally distinct. It has garnered attention for its potential therapeutic benefits, particularly in the context of metabolic disorders like Alzheimer's Disease. Unlike fructose, allulose is absorbed by the body but not metabolized in the same way, offering unique advantages without the negative metabolic effects associated with other sugars.
Enhancing Mitochondrial Function
Mitochondria, the power engine of the cell, play a pivotal role in energy production through oxidative phosphorylation. In Alzheimer's Disease, mitochondrial dysfunction is a critical factor contributing to neuronal degeneration and cognitive decline.
Allulose has been shown to enhance mitochondrial function, which could directly benefit neuronal cells by improving their energy metabolism. This enhancement may occur through several mechanisms, including the upregulation of genes (gene expression) involved in mitochondrial biogenesis and the optimization of mitochondrial efficiency, thereby increasing ATP production.
Enhanced mitochondrial function supports the energy demands of healthy neuronal activity, potentially mitigating some of the metabolic deficits observed in Alzheimer's Disease.
Decreasing Insulin Resistance via Glucagon-Like Peptide 1 (GLP-1) Activation
Allulose's ability to influence insulin sensitivity presents another avenue through which it may exert beneficial effects in Alzheimer's Disease. Insulin resistance in the brain impairs glucose uptake by neurons, exacerbating energy deficits and contributing to cognitive decline.
Allulose has been found to modulate the secretion of glucagon-like peptide 1 (GLP-1), a hormone that enhances insulin sensitivity and glucose tolerance. By stimulating GLP-1 release, allulose may help decrease insulin resistance, facilitating more efficient glucose uptake and utilization by brain cells.
This effect not only supports neuronal health and function but also aligns with strategies aimed at treating Alzheimer's as a metabolic disorder which may be termed “Type 3 diabetes”.
Supporting Proper Glucose Metabolism
Beyond improving insulin sensitivity, allulose possesses properties that directly influence glucose metabolism. Its low glycemic index (essentially zero) means that it does not significantly raise blood glucose levels, a critical factor for individuals with impaired glucose tolerance. Furthermore, allulose has been shown to reduce the absorption of glucose in the intestine, leading to lower postprandial (after-meal) blood glucose levels.
These properties make allulose an attractive dietary component for managing glucose levels, potentially reducing the glucose-related metabolic stress on neurons in Alzheimer's Disease.
Additional Metabolic Benefits
Allulose's metabolic benefits extend beyond its impact on glucose and insulin. Preliminary research suggests that it may also improve lipid metabolism, reducing triglyceride and cholesterol levels, which are often elevated in metabolic disorders.
By addressing these aspects of metabolic health, allulose could contribute to a more comprehensive approach to managing Alzheimer's Disease, addressing not only the direct metabolic impairments in neuronal cells but also the broader metabolic dysregulation associated with the condition.
The multifaceted benefits of allulose ranging from enhancing mitochondrial function and reducing insulin resistance to supporting proper glucose metabolism, offer a promising approach to addressing the metabolic dysfunctions at the heart of Alzheimer's Disease.
As research into allulose and its therapeutic potential continues, it may become a valuable component of integrated treatment strategies aimed at not only improving metabolic health but also mitigating the cognitive decline associated with Alzheimer's.
While further clinical studies are needed to fully understand and optimize allulose's role in treating Alzheimer's, its current profile suggests a novel and hopeful avenue for enhancing neuronal health and function in the context of this challenging disease.
The Role of Candida Fungi in Alzheimer's Disease: A Metabolic Disorder Perspective
The hypothesis linking Candida fungi infection to Alzheimer's Disease introduces a fascinating intersection between infectious agents and neurodegenerative disorders. This connection underscores a possible bidirectional relationship where metabolic dysfunctions in Alzheimer's not only worsen the disease's neuropathology but may also predispose individuals to fungal infections like Candida.
Candida Fungi & Alzheimer's Disease
Candida, a genus of yeast, is a common component of the human microbiota but can become pathogenic under certain conditions, leading to infections that range from superficial mucosal to systemic candidiasis.
Recent research suggests that Candida and other fungi may be more prevalent in the brains of Alzheimer's patients compared to healthy individuals, sparking interest in understanding how these microorganisms might contribute to or exacerbate the disease's pathology.
The presence of Candida in the brain is hypothesized to trigger inflammatory responses, produce neurotoxic compounds, and potentially interfere with normal neuronal function, thereby contributing to the cognitive decline observed in Alzheimer's Disease.
Metabolic Disorders as a Gateway for Candida Infection
The connection between metabolic disorders and increased susceptibility to Candida infections is well-documented in conditions such as diabetes, where alterations in immune function and glucose metabolism create a more favorable environment for fungal growth.
In the context of Alzheimer's Disease, characterized by insulin resistance and impaired glucose metabolism in the brain, similar mechanisms could theoretically increase vulnerability to Candida colonization and infection.
High blood sugar levels can impair immune function, reducing the body's ability to fight off infections, including those caused by Candida. Additionally, the compromised metabolic state of neuronal cells in Alzheimer's could further exacerbate the impact of Candida on brain health, potentially accelerating disease progression.
The Impact of Candida on Neuronal Health & Alzheimer's Progression
The presence of Candida in the brain may have direct and indirect effects on neuronal health and Alzheimer's progression. Direct effects could include the production of neurotoxic metabolites by Candida that damage neurons, while indirect effects might involve the activation of chronic inflammatory pathways that are already implicated in Alzheimer's pathology.
These inflammatory responses can lead to further neuronal damage and exacerbate the accumulation of amyloid-beta and tau proteins, key hallmarks of Alzheimer's Disease.
Addressing Candida Infections in Alzheimer's: Therapeutic Implications
Understanding the role of Candida in Alzheimer's opens potential therapeutic avenues focusing on antimicrobial and antifungal strategies as part of a broader treatment regimen.
Antifungal medications like fenbendazole, traditionally used in veterinary medicine but also explored for its antifungal properties in humans, could offer a novel approach to reducing Candida load and its detrimental effects on the brain.
However, any therapeutic strategy targeting Candida would need to be carefully integrated with treatments addressing the underlying metabolic dysfunctions and neuropathological features of Alzheimer's to ensure a comprehensive approach to managing the disease.
The exploration of Candida fungi's role in Alzheimer's Disease, particularly within the framework of metabolic disorders, highlights a complex interplay between infectious agents, metabolic health, and neurodegeneration.
The potential for Candida to exacerbate Alzheimer's pathology through direct and indirect mechanisms underscores the need for a multifaceted treatment strategy that addresses both the metabolic underpinnings of Alzheimer's and the possible contributions of microbial infections to its progression.
As research in this area advances, a deeper understanding of these connections may lead to more effective interventions aimed at mitigating the impact of both metabolic dysfunction and infectious agents on Alzheimer's Disease.
Fenbendazole: Unveiling Its Anti-Candida Properties in the Context of Alzheimer's Disease
Fenbendazole, a benzimidazole antifungal medication commonly used in veterinary medicine, has emerged as a compound of interest for its potential anti-Candida properties and implications for Alzheimer's Disease treatment.
With the growing recognition of the role of Candida and other fungi in the progression of Alzheimer's, particularly in the context of metabolic dysfunctions, the exploration of effective antifungal treatments like fenbendazole becomes increasingly relevant.
Mechanism of Action of Fenbendazole
Fenbendazole works by disrupting the microtubule networks within fungal cells, impairing their ability to divide and grow. Specifically, it binds to beta-tubulin, an essential protein for microtubule formation, thereby inhibiting the polymerization of microtubules. This action results in the disruption of essential cellular processes, leading to the death of the fungal cell.
While fenbendazole's primary use has been in treating parasitic infections in animals, its mechanism of action suggests a broad-spectrum potential that includes efficacy against fungal pathogens like Candida.
Fenbendazole's Anti-Candida Properties
Research into fenbendazole's antifungal properties has shown promise in its ability to inhibit the growth of Candida species, including strains resistant to other antifungal medications.
This is particularly significant in the context of Alzheimer's Disease, where Candida's presence in the brain may contribute to the disease's progression through mechanisms such as inflammation and neurotoxicity.
By effectively reducing Candida load, fenbendazole could mitigate these adverse effects, potentially slowing or altering the course of Alzheimer's pathology.
The Implications for Alzheimer's Disease Treatment
The potential use of fenbendazole in Alzheimer's Disease treatment strategies is multifaceted. Beyond its direct antifungal effects, fenbendazole may also offer anti-inflammatory benefits, reducing the neuroinflammation associated with both Alzheimer's pathology and Candida infection.
Moreover, given the link between metabolic dysfunction and increased susceptibility to fungal infections, fenbendazole could play a role in a more comprehensive treatment approach that addresses both the metabolic and infectious aspects of Alzheimer's Disease.
Tauopathy & Hyperphosphorylation in Alzheimer's Disease: Understanding the Core Pathology
Tauopathy, characterized by the accumulation of abnormally hyperphosphorylated tau protein in the brain, stands as a central pathology in Alzheimer's Disease.
This process leads to the formation of neurofibrillary tangles (NFTs), a hallmark of Alzheimer's, which disrupts neuronal function and contributes significantly to cognitive decline.
Understanding the mechanisms of tauopathy and hyperphosphorylation is crucial for developing targeted therapies aimed at mitigating these pathologies.
The Role of Tau Protein in Neuronal Health
Tau protein plays a critical role in stabilizing microtubules within axons, essential for maintaining neuronal structure and facilitating intracellular transport.
Under normal conditions, tau protein is phosphorylated at several sites, a process that regulates its binding to microtubules and ensures neuronal health and functionality. However, in Alzheimer's Disease, an imbalance in the phosphorylation and dephosphorylation processes leads to the pathological hyperphosphorylation of tau.
Hyperphosphorylation & the Formation of Neurofibrillary Tangles (NFTs)
Hyperphosphorylation of tau reduces its affinity for microtubules, leading to microtubule destabilization and impaired axonal transport. The hyperphosphorylated tau proteins aggregate to form paired helical filaments, which eventually coalesce into neurofibrillary tangles within neurons (plaques).
These tangles contribute to cell death and the progressive neurodegeneration observed in Alzheimer's Disease. The spread of NFTs throughout the brain correlates with the severity of cognitive impairment, making the control of tau hyperphosphorylation a key target for Alzheimer's therapies.
The Impact of Tauopathy on Alzheimer's Disease Progression
Tauopathy exacerbates Alzheimer's Disease progression through several mechanisms. Beyond disrupting microtubule stability and axonal transport, NFTs are also implicated in synaptic dysfunction and neuronal loss. Furthermore, tau aggregates can induce neuroinflammatory responses, further damaging brain tissue and exacerbating neurodegeneration.
The multifaceted impact of tauopathy on neuronal health highlights the need for therapeutic strategies that can effectively target and mitigate this pathology.
Targeting Tau Hyperphosphorylation: Therapeutic Approaches
Given the central role of tau hyperphosphorylation in Alzheimer's pathology, developing therapies that can prevent, reverse, or mitigate tauopathy is of paramount importance.
Potential strategies include kinase inhibitors that reduce tau phosphorylation, tau aggregation inhibitors that prevent the formation of NFTs, and immunotherapies designed to clear tau aggregates from the brain. Each approach targets a different aspect of tau pathology, offering potential pathways to slow or halt the progression of Alzheimer's Disease.
The Future of Tauopathy Treatment in Alzheimer's Disease
Advances in understanding the molecular mechanisms underlying tauopathy and hyperphosphorylation are paving the way for novel therapeutic interventions.
Ongoing research and clinical trials continue to explore the efficacy and safety of these potential treatments, aiming to offer new hope for individuals affected by Alzheimer's Disease.
The complexity of tauopathy underscores the need for a multifaceted therapeutic approach, combining targeted treatments with interventions addressing the broader spectrum of Alzheimer's pathology for maximum efficacy.
Ivermectin & Its Role in Preventing Hyperphosphorylation in Alzheimer's Disease
Ivermectin, a widely used antiparasitic agent, has recently garnered attention for its potential neuroprotective properties, particularly its ability to prevent the hyperphosphorylation of tau protein, a key pathological feature of Alzheimer's Disease.
Ivermectin: Mechanism of Action Against Tau Hyperphosphorylation
Ivermectin has been observed to interfere with several molecular pathways that could be beneficial in the context of reducing the deleterious effects of Alzheimer's Disease. Specifically, it has been suggested that ivermectin can modulate the activity of certain enzymes involved in the phosphorylation of tau protein.
By inhibiting specific kinases responsible for tau hyperphosphorylation, ivermectin may help maintain tau protein in a more physiologically normal phosphorylated state, thereby preventing the formation of neurofibrillary tangles and subsequent neuronal damage.
Potential Neuroprotective Effects of Ivermectin
Beyond its direct effects on tau phosphorylation, ivermectin may exert additional neuroprotective effects that could be beneficial in Alzheimer's Disease. These include reducing neuroinflammation, modulating the immune response within the central nervous system, and potentially facilitating the clearance of amyloid-beta plaques.
The combination of these effects suggests that ivermectin could offer a multifaceted approach to slowing the progression of Alzheimer's Disease by targeting several key aspects of the disease pathology.
Evidence Supporting Ivermectin's Efficacy in Alzheimer's Disease
Research into ivermectin's efficacy in Alzheimer's Disease is still in the early stages, with most studies conducted in vitro (in cell cultures) or in vivo (in animal models).
These studies have shown promising results, with ivermectin reducing tau hyperphosphorylation and ameliorating other markers of Alzheimer's pathology. However, translating these findings into clinical practice will require rigorous clinical trials to evaluate the safety, optimal dosing, and efficacy of ivermectin in human patients with Alzheimer's Disease.
The complexity of Alzheimer's Disease, characterized by its multifactorial etiology involving metabolic dysfunction, tauopathy, and potential infectious components, necessitates a comprehensive and integrated approach to treatment.
Integrating Therapeutic Strategies
The integration of treatments targeting metabolic dysfunction, infectious components, and tauopathy presents a comprehensive approach to Alzheimer's Disease management. Such a strategy acknowledges the complexity of AD and leverages the synergistic potential of combining treatments to address its multifaceted pathology.
A critical aspect of this integrated approach is the careful consideration of dosing, timing, and potential interactions between these compounds to maximize their therapeutic benefits while minimizing risks.
Alzheimer's Disease represents one of the most challenging neurodegenerative disorders, with its complex interplay of metabolic, infectious, and pathological mechanisms contributing to its progression.
The exploration of allulose, fenbendazole, and ivermectin as potential treatments offers hope for addressing the diverse aspects of AD pathology. Allulose's metabolic benefits, fenbendazole antifungal properties, and ivermectin's ability to inhibit tau hyperphosphorylation, each target different underlying factors of the disease.
Integrating these approaches could pave the way for more effective and comprehensive treatment strategies, potentially slowing or even halting the progression of Alzheimer's Disease. Approaches which “Biden’s” medical team should have considered long ago, rather than pumping him full of toxic cocktails comprised of various ineffectual dementia meds and methamphetamine stimulants like Adderall that merely allow for short bursts of incomprensible babble and angry outbursts.
They want you dead.
Do NOT comply.















What about the effect of aluminium in the brain. There is a scientist by the name of Dr. Christopher Exley who has been doing a lot of research on this neurotoxin. It seems that this element is in greater quantity in the brain's of dementia patients. This still needs more research, but industry including the Pharma groups keep blocking funding to said research because they use this element in their products. Also aluminium is now in the air we breathe from geoengineering.
Jan
Wow! To make an oversimplification, all of these terrible afflictions seem to be parasitic in nature, certainly Candida qualifies, and all compounds that disrupt the metabolism of and kill parasites are effective against them...Allopathic Medicine has been barking up the wrong tree for a century or more...