
The Promise of Fenbendazole and Ivermectin: A Comparison to New "Cancer-Stopping Pill" AOH1996 in Targeting Transcription Replication
A team of U.S. researchers claim to have developed a new anticancer pill that “annihilates” tumors.
That’s according to their preclinical research findings, published Aug. 1 in the Cell Chemical Biology journal.
Titled “Small Molecule Targeting of Transcription-Replication Conflict for Selective Chemotherapy,” the study found that a drug the researchers developed was able to selectively disrupt DNA replication and repair in cancer cells.
The drug is called AOH1996. It was named after Anna Olivia Healey, who was born in 1996 and died of a rare form of cancer called neuroblastoma when she was 9 years old.
Linda Malkas, a senior author of the new study based at the City of Hope, has been developing the drug over the past two decades.
City of Hope is a private, non-profit clinical research center, hospital, and graduate school in Duarte, California. It’s one of the largest cancer research and treatment organizations in the United States.
According to a press release from City of Hope, the AOH1996 “appears to annihilate all solid tumors.”
Now that research has demonstrated promising results in cell and animal models, the new drug is being tested in humans in a Phase 1 clinical trial, Ms. Malkas said in a statement.
Unique Mechanism
The preclinical study explains how AOH1996 targets a cancerous version of the protein, called proliferating cell nuclear antigen (PCNA).
The cancerous variant of PCNA “is critical in DNA replication and repair” of all cancerous tumors, but the new drug AOH1996 inhibits PCNA, the City of Hope noted in its release.
“Data suggests PCNA is uniquely altered in cancer cells, and this fact allowed us to design a drug that targeted only the form of PCNA in cancer cells,” Ms. Malkas said.
“PCNA is like a major airline terminal hub containing multiple plane gates,” she explained. “Our cancer-killing pill [AOH1996] is like a snowstorm that closes a key airline hub, shutting down all flights in and out only in planes carrying cancer cells.”
Given orally at a certain dose, AOH1996 “suppresses tumor growth but causes no discernable side effect,” authors wrote in the study.
Most other targeted cancer therapies focus on a single pathway, which allows cancer cells to mutate and eventually become resistant to the therapy, Ms. Malkas noted. The unique mechanism of AOH1996 that enables it to target the mutated versions of PCNA sets it apart from other currently available therapies.
Ms. Malkas said results “have been promising” and they suggest that AOH1996 “can suppress tumor growth as a monotherapy or combination treatment in cell and animal models without resulting in toxicity.”
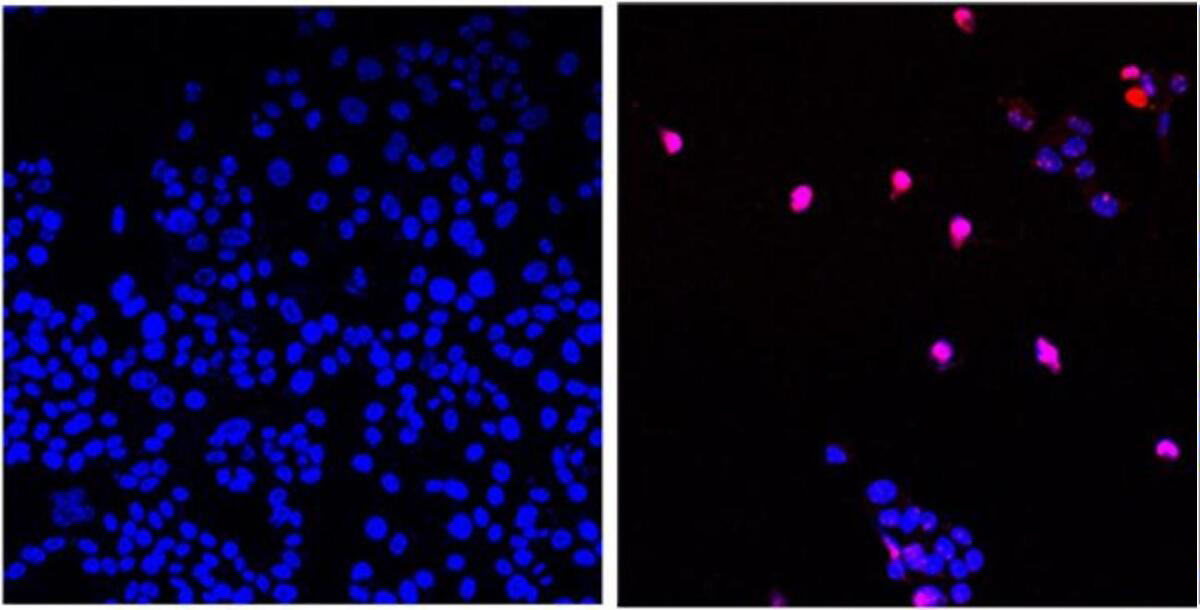
Introduction:
As the search for effective and selective anticancer therapies continues, Fenbendazole and Ivermectin have emerged as promising candidates, offering unique mechanisms of action and established safety profiles. Here we compare the anticancer properties of Fenbendazole and Ivermectin to AOH1996, a small molecule PCNA inhibitor, in the context of targeting transcription replication conflicts for cancer treatment.
Established Safety Profiles and Clinical Applicability:
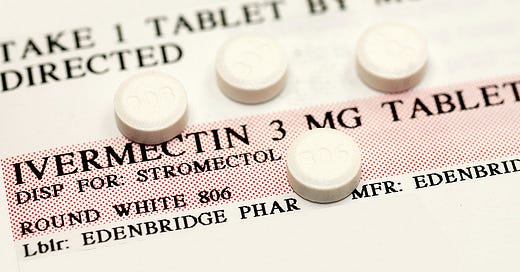
Fenbendazole and Ivermectin have demonstrated their safety and efficacy in treating parasitic infections in both veterinary and human medicine. These drugs have been used extensively, and have a well-established safety record. Their repurposed status grants them a significant advantage over novel compounds like AOH1996, which often require extensive safety evaluations before clinical translation. This streamlined path to clinical use for Fenbendazole and Ivermectin accelerates their potential to be readily available to patients, making them attractive options for cancer treatment.
Broad-Spectrum Mechanisms of Action:
Unlike AOH1996, which primarily targets PCNA and its interaction with RNA polymerase II's largest subunit, Fenbendazole and Ivermectin exhibit diverse mechanisms of action. Fenbendazole disrupts the microtubular network, inducing cell cycle arrest and apoptosis in cancer cells. Similarly, Ivermectin upregulates the tumor suppressor protein P53, leading to enhanced cancer cell death.
These multi-targeted effects may offer advantages over targeted therapies, as they can potentially address heterogeneous cancer populations with varied genetic backgrounds, reducing the likelihood of resistance.
Cost-Effectiveness and Accessibility:
Fenbendazole and Ivermectin are generic medications, making them cost-effective options for cancer treatment, particularly in low-resource settings. In contrast, targeted therapies and novel compounds like AOH1996 can be expensive, and may not be accessible to all patients due to financial constraints. The affordability and widespread availability of Fenbendazole and Ivermectin contribute to their appeal as potential treatments for a broader patient population.
Synergistic Effects with Conventional Therapies:
By targeting different cellular processes, such as microtubules and P53, these repurposed drugs lead to improved treatment outcomes. AOH1996's mechanisms are highly selective, and efficacy can vanish with a single mutation. (Resistance to AOH1996 can be conferred by mutations in RPB1's PCNA-binding region, which impairs the interaction between RPB1 and PCNA, making cancer cells less susceptible to the effects of the inhibitor. Resistance caused by random mutations is a potential problem with many targeted therapeutic approaches, including the inhibition of PCNA described in the AOH1996 paper. When cancer cells are exposed to selective pressures such as from a targeted drug, some cells may acquire genetic changes through random mutations that confer resistance to the drug's effects.)
Fenbendazole and Ivermectin offer unique advantages over AOH1996 as potential anticancer therapies. Their established safety profiles, diverse mechanisms of action, cost-effectiveness, and potential for synergistic use with conventional treatments make them attractive candidates for further research and clinical trials. As research progresses, these repurposed drugs may prove to be valuable additions to the therapeutic arsenal against cancer, providing hope for more accessible and effective treatments for patients worldwide.
Synergistic pairing of ivermectin and fenbendazole found HIGHLY EFFECTIVE at preventing and treating cancer
This Substack has recently written about the wonder drugs Ivermectin and Fenbendazole: As a combination therapy these two drugs offer a highly synergistic approach to curing a wide range of ailments from slow kill bioweapon injection damage to prior-based diseases to (turbo) cancers, etc.
The anticancer properties of these repurposed drugs are far more promising than the targeted therapeutic approach taken with compounds such as AOH1996.
Do NOT comply.
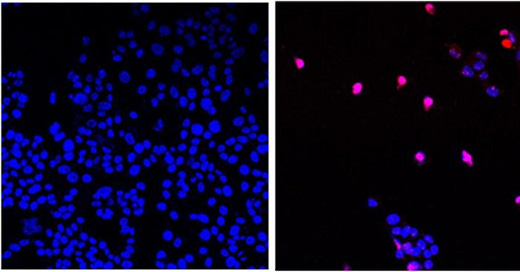











To me, it sounds like a way to profit from illness that is being manufactured. The increase of cancers could be attributed to the covid jab and other so called vaccines, food and consumer product additives, factory pollution and garbage waste, and wildfires. Plus, I am very suspicious of a relatively new drug being used. It is amazing how long-term side effects slip under the radar.
I am waiting for a post from 2nd exploring the possibility that these drugs are effective because of a parasitical aspect of Cancer.
I hope it seems an interesting enough topic to explore.